
A team of bioengineers at the Wyss Institute for Biologically Inspired Engineering at Harvard University and the Harvard John A Paulson School of Engineering and Applied Sciences (SEAS) has developed a mechanically active adhesive that functions as a soft robotic device that can prevent, and support the recovery from, muscle atrophy.
When muscles aren’t exercised enough, they can begin to waste away or atrophy. Mechanotherapy such as massage slow or reverse this process; however, it’s unclear whether stretching and contracting muscles by external means can also be effective. So far, two major challenges have prevented such studies: limited mechanical systems capable of evenly generating stretching and contraction forces along the length of muscles, and inefficient delivery of these mechanical stimuli to the surface and into the deeper layers of muscle tissue.
The new device developed by the Wyss Institute team, dubbed MAGENTA (‘mechanically active gel-elastomer-nitinol tissue adhesive’), solves both of these problems. ‘With MAGENTA, we developed a new integrated multi-component system for the mechanostimulation of muscle that can be directly placed on muscle tissue to trigger key molecular pathways for growth,’ said David Mooney, a Wyss Founding Core Faculty member and the Robert P Pinkas family professor of bioengineering at SEAS. ‘While the study provides first proof-of-concept that externally provided stretching and contraction movements can prevent atrophy in an animal model, we think that the device’s core design can be broadly adapted to various disease settings where atrophy is a major issue.’
One of MAGENTA’s major components is an engineered spring made from nitinol, a type of metal known as a ‘shape memory alloy’ (SMA) that enables MAGENTA’s rapid actuation when heated to a certain temperature. The researchers actuated the spring by electrically wiring it to a microprocessor unit that allows the frequency and duration of the stretching and contraction cycles to be programmed.
The other components of MAGENTA are an elastomer matrix that forms the body of the device and insulates the heated SMA and a ‘tough adhesive’ that enables the device to be firmly adhered to muscle tissue. In this way, the device is aligned with the natural axis of muscle movement, transmitting the mechanical force generated by the SMA deep into the muscle. Mooney’s group is advancing MAGENTA as one of several tough gel adhesives with functionalities tailored to various regenerative applications across multiple tissues.
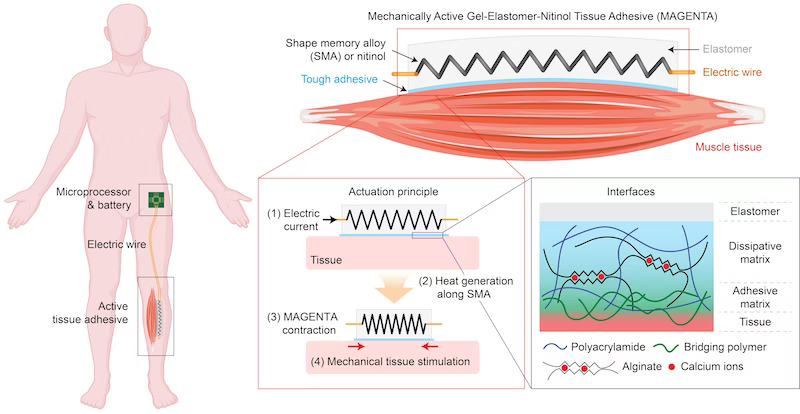
After designing and assembling the MAGENTA device, the team tested its muscle-deforming potential, first in isolated muscles ex vivo and then by implanting it on one of the major calf muscles of mice. The device didn’t induce any serious signs of tissue inflammation or damage, and exhibited a mechanical strain of about 15 per cent on muscles, which matches their natural deformation during exercise.
Next, to evaluate its therapeutic efficacy, the researchers used an in vivo model of muscle atrophy by immobilising a mouse’s hind limb in a tiny cast-like enclosure for up to two weeks after implanting the MAGENTA device on it. ‘While untreated muscles and muscles treated with the device but not stimulated significantly wasted away during this period, the actively stimulated muscles showed reduced muscle wasting,’ said Wyss technology development fellow Sungmin Nam. ‘Our approach could also promote the recovery of muscle mass that already had been lost over a three-week period of immobilisation, and induce the activation of the major biochemical mechano-transduction pathways known to elicit protein synthesis and muscle growth.’
In a previous study, regulated cyclical compression (as opposed to stretching and contraction) of acutely injured muscles, using a different soft robotic device, was found to reduce inflammation and enable the repair of muscle fibres. In the new study, Mooney’s team asked whether those compressive forces could also protect from muscle atrophy. However, when they directly compared muscle compression using the previous device to muscle stretching and contraction using the MAGENTA device, only the latter had clear therapeutic effects in the mouse atrophy model. ‘There is a good chance that distinct soft robotic approaches with their unique effects on muscle tissue could open up disease- or injury-specific mechano-therapeutic avenues,’ said Mooney.
To further expand the possibilities of MAGENTA, the team explored whether the SMA spring could also be actuated by laser light, which would make the approach essentially wireless, broadening its therapeutic usefulness. They demonstrated that an implanted MAGENTA device without any electric wires could function as a light-responsive actuator and deform muscle tissue when irradiated with laser light through the overlying skin layer.
While laser actuation didn’t achieve the same frequencies as electrical actuation, and fat tissue seemed to absorb some laser light, the researchers believe that the demonstrated light sensitivity and performance of the device could be further improved. ‘The general capabilities of MAGENTA and fact that its assembly can be easily scaled from millimetres to several centimetres could make it interesting as a central piece of future mechanotherapy, not only to treat atrophy, but perhaps also to accelerate regeneration in the skin, heart and other places that might benefit from this form of mechano-transduction,’ said Nam.
The research has been published in Nature Materials.


