
Researchers at Imperial College London have developed organic electrode materials that could provide the solution to sustainable energy storage.
Recently, concerns have been growing about the high cost, toxicity, environmental pollution and end-of-life recycling of lithium-ion batteries, which currently dominate the market when it comes to portable electronics and electric vehicles. Sustainable organic electrode materials could potentially replace these conventional inorganic materials, but so far they’ve been limited by performance challenges.
Now, an international team led by Qilei Song from the Department of Chemical Engineering at Imperial College London has developed a new type of organic electrode material whose performance in Li-ion batteries has proved promising.
Conventional lithium-ion batteries typically use inorganic electrode materials such as lithium cobalt oxide and lithium manganese oxide, which are damaging to the environment and are in limited supply. Song’s team has developed organic electrode materials that integrate redox-active organic molecules, which release and store energy, into long-chain polymers. The resulting polymer particles are dissolved and mixed with carbon additives to make battery electrodes.
This newly designed polymer electrode material has improved stability and addresses existing problems with organic electrode molecules, including the loss of storage capacity over time and slow ion transport and electron transfer – the critical aspect responsible for energy deployment and charging in batteries.
‘To solve the scientific challenges associated with organic electrode materials, we developed new polymer electrode materials that combine solution-processability, redox activity, and sub-nanometre pores,’ said the study’s lead author, Anqi Wang (pictured below). ‘Our molecular design approach is a synergistic combination of organic electrode materials and porous polymers developed in recent decades.’

The polymer electrode materials possess intrinsic sub-nanometre pores that enable fast Li-ion transport during battery operation. The generation of these sub-nanopores is a direct consequence of the materials’ unique macromolecular chain structures, which are highly rigid and awkwardly shaped.
The polymers’ other unique property is their solution processability, which means that they can be dissolved into an organic solvent and then processed into any form or shape as a composite or a stand-alone material. This adds greater versatility and simplicity to their use as battery electrodes.
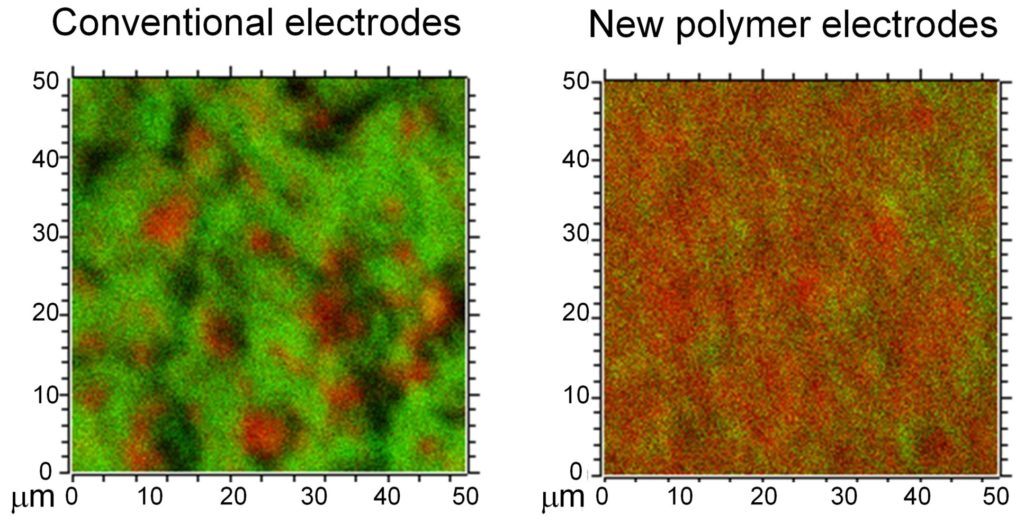
The solution processing method that the team developed, in which they dissolved the polymers in solvent and mixed it with electron-conductive carbon additives, also showed significantly improved mixing and contact between polymers and carbon additives, including carbon nanotubes.
Such control over interfaces could promote homogeneous current distributions within the electrode, something that has been difficult to achieve in conventional electrode materials, but is key to ensuring the battery has a long lifetime. The newly developed battery electrodes demonstrated stable cycling performance without any apparent capacity decay over more than 1,000 cycles of charging and discharging.
‘The solution processing technique is suitable for integration with the existing battery electrode manufacturing processes already used in industry at large scale,’ said Rui Tan, who helped develop the electrodes.
Next, the researchers will apply the latest machine learning techniques to screen a large database of organic building blocks to develop the next generation of redox electrode materials with fast conduction of both metal ions and electrons.
The molecular design approach is also applicable to materials for other energy storage systems, such as sodium-ion batteries and redox flow batteries. The team is seeking opportunities to collaborate with other research teams to explore these applications, and to work with the Faraday Institution to investigate the degradation and manufacturing of these polymer electrode materials.
The study has been published in the Journal of the American Chemical Society.



