Engineers at the University of Illinois Chicago have built a machine that captures carbon from flue gas and converts it to ethylene.
The device is the first to integrate a carbon-capture system with an ethylene conversion system. While the system runs on electricity, it still manages to remove more carbon from the environment than it generates – making it net-negative on carbon emissions.
Among manufactured chemicals, ethylene ranks third for carbon emissions after ammonia and cement. It’s used to create plastic products for the packaging, agricultural and automotive industries and to produce chemicals used in antifreeze, medical sterilisers and vinyl siding for houses, among other things.
‘This is the first demonstration of a net-negative, all-electric integrated system to capture carbon from pollutants and create a highly valuable resource,’ said Meenesh Singh, a UIC assistant professor in the Department of Chemical Engineering. ‘There is an urgent need to develop efficient technologies for integrated carbon capture and conversion to sustainably produce net-negative fuels. Currently, integrated carbon capture and conversion systems are highly energy-intensive and work in a discontinuous cycle of carbon dioxide capture and reduction. Efficiently integrating carbon capture with the conversion system eliminates the need for transportation and storage, and thereby increasing its energy efficiency.’
To capture carbon from the air or flue gas, Singh’s lab modified a standard artificial-leaf system with inexpensive materials to include a water gradient – a dry side and a wet side – across an electrically charged membrane. On the dry side, an organic solvent attaches to available carbon dioxide to produce a concentration of bicarbonate, or baking soda, on the membrane. As the bicarbonate builds up, its negatively charged ions are pulled across the membrane towards a positively charged electrode in a water-based solution on the membrane’s wet side. The liquid solution dissolves the bicarbonate back into carbon dioxide, so that it can be released and harnessed for CO2 conversion.
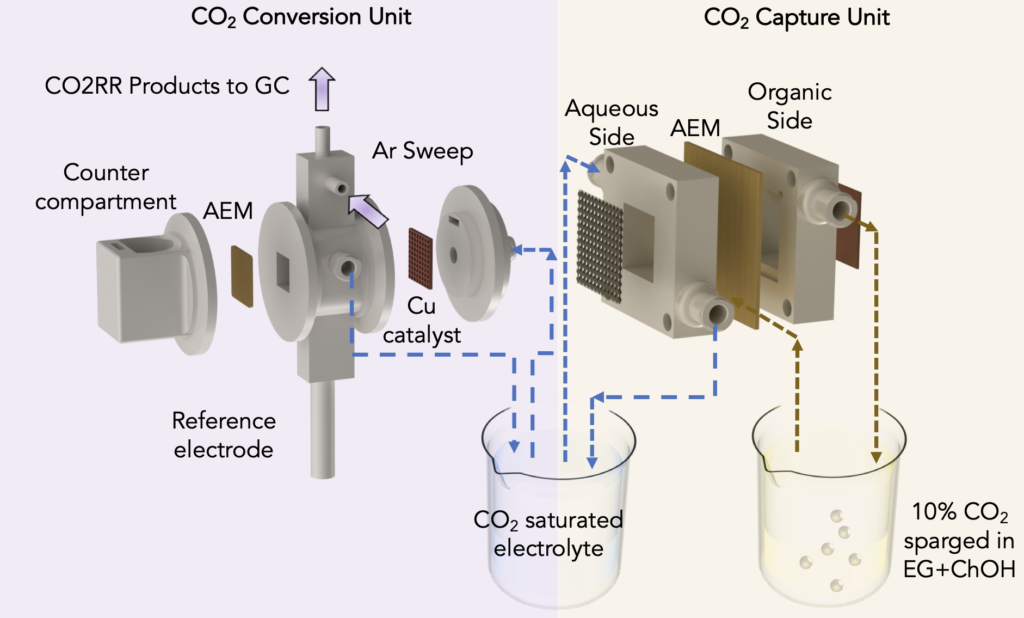
To convert the captured CO2 to ethylene, Singh and his colleagues used a second system in which an electric current is passed through a cell divided in two by a membrane. Half of the cell is filled with carbon dioxide captured from a carbon-capture system, the other half with a water-based solution. An electrified catalyst draws charged hydrogen atoms from the water molecules into the other half of the cell, where they combine with charged carbon atoms from the carbon dioxide molecules to form ethylene.
The UIC researchers integrated the two systems by feeding the captured-CO2 solution to the carbon-conversion system and recycling it back. The closed-loop recycling of solution ensures a constant supply of carbon dioxide from flue gas and its conversion to ethylene.
To test their integrated system, the researchers implemented a 100-square-centimetre bipolar membrane electrodialysis unit to capture carbon dioxide from the flue gas and hydraulically connected it to the one-square-centimetre electrolysis cell to produce ethylene.
They were able to test the system continuously, 24 hours per day for seven days. The system was not only stable the entire time, it also captured carbon at a rate of 24 grams per day and produced ethylene at a rate of 188 milligrams per day.
‘In the journey to make ethylene production green, this is a potential breakthrough,’ Singh said. ‘Our next step is to scale up the integrated carbon capture and conversion system to produce ethylene at higher rates — a rate of one kilogram per day and capture carbon at a rate higher than kilograms per day.’
The system uses a modular, stackable design that allows it to be easily scaled up and down.
The research has been published in Energy & Environmental Science.



